- Detection - Measurement
- Flow, Pressure and Level Measurements
- Vacuum leak tester
- Bonfiglioli Engineering S.r.l.

- Products
- Catalogs
- News & Trends
- Exhibitions
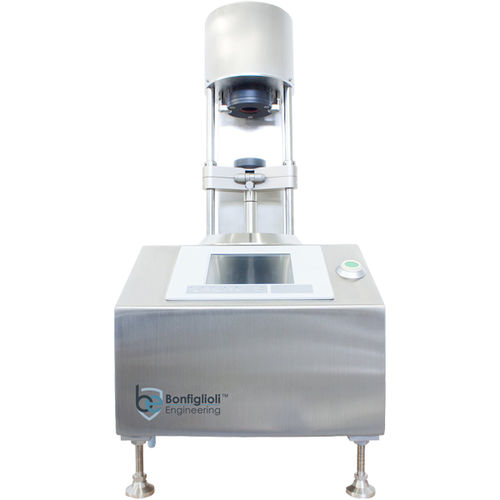
Vacuum leak tester LF-400Apressurefor containersfor aerosol cans
Add to favorites
Compare this product
Characteristics
- Type
- vacuum, pressure
- Applications
- for containers, for aerosol cans
- Other characteristics
- compact
Description
Off-line Machine for Non-Invasive, Non-Destructive Integrity Inspection for laboratory and in process control applications for filled Aerosol Can.
Highlights
-Quick and sensitive test
-Compact & maintenance free
-Vacuum testing
-Fast, reliable and repeatable results
-Applicable to any type of pharmaceutical containers
-Highly functional, intuitive HMI
Technical Features
-Container Application: Filled Aerosol, Monobloc, MDI
-Products: Liquid
-Container Dimensions: From Ø8 X 25 mm (H) to Ø65 x 300 mm (max)
-Speed: Up to 4 Cpm
-Technology: CCIT
-Inspection features: Non-Invasive, Non-Destructive CCIT based on Vacuum Decay Method
-Inspection Capabilities: Microleaks detection
Additional Benefits
-Easy to clean – no hidden corners.
-Quick format changeover
-Low power consumption.
-Real time display of testing cycle diagrams, statistical raw data
-Easy, quick and safe remote access
-Barometric Compensation system to avoid any vacuum level reading variations
-Storage of records: maintenance, production, alarms
Technology
-Container Closure Integrity Testing is a non-destructive measurement technology based on Vacuum Decay Method.
-Measurement system comprises applying a pressure differential into an airtight testing group enclosing the container.
-The test objective is to detect container leakages by measuring the reached pressure level as well as the pressure change over test time.
Quality Assurance
-Equipment test method refers to:
-Approved industry standard “ASTM F2338-09”: “Standard Test Method for Non-Destructive Detection of Leaks in Packages”
Other Bonfiglioli Engineering S.r.l. products
Metal Cans and Aerosols
Related Searches
- Leak testing device
- Pressure leak testing device
- Automatic leak testing machine
- Packaging leak testing device
- Vacuum leak testing machine
- Bottle leak testing machine
- Vessel leak testing device
- Compact leak tester
- Pneumatic leak test bench
- In-line leak tester
- Empty container leak tester
- Flow leak testing device
- High-speed leak tester
- Tabletop leak tester
- Multi-head leak tester
- Aerosol can leak tester
- Container leak tester
- Full container leak tester
- Mechanical leak test bench
- Rotary leak tester
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.








