
- Products
- Catalogs
- News & Trends
- Exhibitions
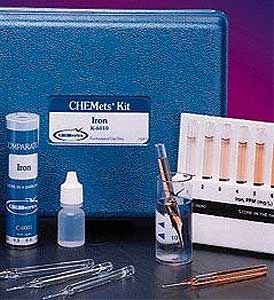
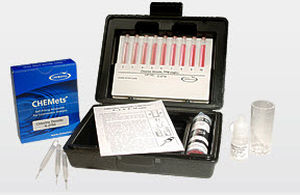
Alkalinity test kit
Add to favorites
Compare this product
Characteristics
- Test type
- alkalinity
Description
The alkalinity of water is a measurement of its buffering capacity. Alkalinity of natural waters is typically a combination of bicarbonate, carbonate, and hydroxide ions. Sewage and wastewaters usually exhibit higher alkalinities due to the presence of silicates and phosphates.
Alkalinity inhibits corrosion in boiler and cooling waters. It is also measured as a means of controlling water and wastewater treatment processes or the quality of various process waters.
CHEMetrics' total alkalinity tests determine total or M alkalinity using a hydrochloric acid titrant and a bromocresol green/methyl red indicator. The end point of the titration occurs at pH 4.5. Results are expressed as ppm (mg/L) CaCO3.
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.