- Products
- Catalogs
- News & Trends
- Exhibitions
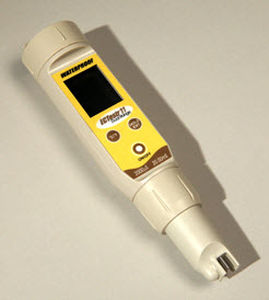
Chlorine dioxide measuring instrument portable
Add to favorites
Compare this product
Characteristics
- Applications
- chlorine dioxide
- Configuration
- portable
Description
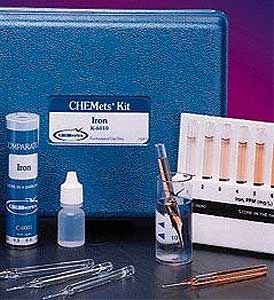
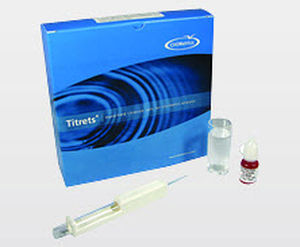
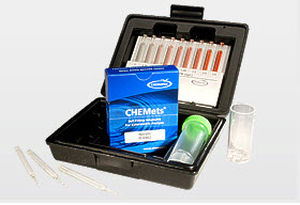
Method
References: USEPA Methods for Chemical Analysis of Water and Wastes, Method 330.5 (1983). APHA Standard Methods, 20th ed., Method 4500- ClO2 D (1998) and 21st ed., Method 4500-Cl G (2005).
Chlorine dioxide is used as an oxidizing microbiocide in industrial cooling water treatment, the dairy industry, the meat industry, and many other food and beverage industry applications. It is used as a bleaching agent in the pulp and paper industry, and as a disinfectant in municipal water treatment. Industrial waste treatment facilities use chlorine dioxide because of its selectivity for certain compounds, including phenols, sulfides, cyanides, thiosulfates, and mercaptans. The oil and gas industry uses chlorine dioxide for downhole applications and as a stimulation enhancement additive. The Maximum Residual Disinfectant Level for chlorine dioxide is 0.8 mg/L in drinking water.
In the standard DPD methodology, chlorine dioxide reacts with DPD (N, N-diethyl-p-phenylenediamine) to form a pink product. Interference from free Cl2 is prevented (up to 6 ppm Cl2) by the addition of glycine to the sample. Results are expressed as ppm (mg/L) ClO2.
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.