- Products
- Catalogs
- News & Trends
- Exhibitions
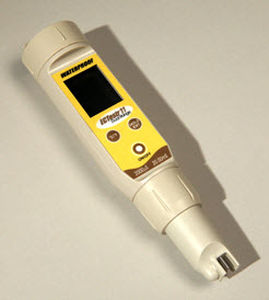
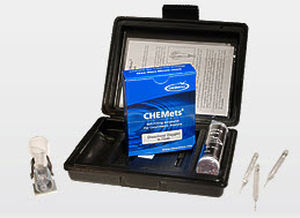
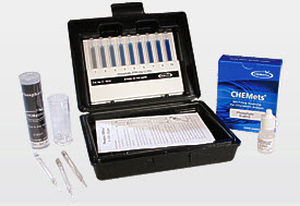
Nitrate analyzer waterconcentrationportable

Add to favorites
Compare this product
Characteristics
- Measured entity
- nitrate, water
- Measured value
- concentration
- Configuration
- portable
Description
Methods
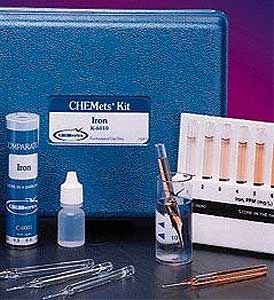
Nitrate is the most completely oxidized form of nitrogen. It is formed during the final stages of biological decomposition, either in wastewater treatment facilities or in natural water supplies. Low-level nitrate concentrations may be present in natural waters. However, a Maximum Contaminant Level of 10 ppm nitrate-nitrogen has been established for drinking water by the USEPA.
The Cadmium Reduction Method
References: ASTM D 3867-09, Nitrate-Nitrite in Water, Test Method B. APHA Standard Methods, 21st ed., Method 4500-NO3- E (2005). USEPA Methods for Chemical Analysis of Water and Wastes, Method 353.3 (1983).
Nitrate is reduced to nitrite using cadmium as the reducing agent. The resulting nitrite concentration is then determined colorimetrically. This method is applicable to drinking and surface waters, as well as domestic and industrial wastes. Nitrite will interfere with this test. Results are expressed as ppm (mg/L) NO3-N or NO3.
The Zinc Reduction Method
References: ASTM D 3867-09, Nitrate-Nitrite in Water, Test Method B. APHA Standard Methods, 21st ed., Method 4500-NO3- E (2005). USEPA Methods for Chemical Analysis of Water and Wastes, Method 353.3 (1983). Nelson, J.L., Kurtz, L.T., and R.H. Bray, Rapid Determination of Nitrates and Nitrites. Anal. Chem., V26, p. 1081-1082, (1954).
Related Searches
- Gas analyser
- Concentration analyser
- Liquids analyser
- Dust analyzer
- Portable analyser
- Water analyser
- Temperature analyser
- Trace analyser
- Carbon dioxide analyser
- PH meter
- Hydrogen analyser
- Conductivity meter
- Portable pH meter
- Chlorine analyser
- Photometer
- Process pH meter
- Nephelometer
- Ozone analyser
- Portable conductivity meter
- Water analysis photometer
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.