- Products
- Catalogs
- News & Trends
- Exhibitions
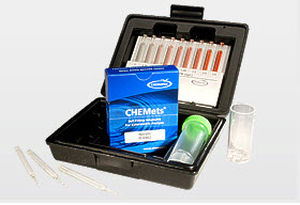
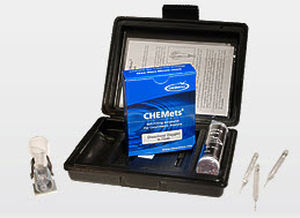
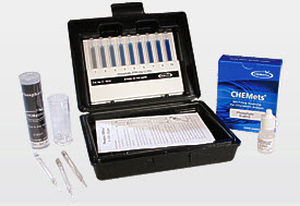
Water analyzer ozoneportable
Add to favorites
Compare this product
Characteristics
- Measured entity
- water, ozone
- Configuration
- portable
Description
Methods
Ozone is a strong oxidizing agent and is used as an alternative to chlorine as a biocide in the disinfection of drinking water. Ozone is used to remove odor, decolorize, and to control algae and other aquatic growths.
Ozone is also used in various disinfectant/sterilization processes in the food & beverage and pharmaceutical industries.
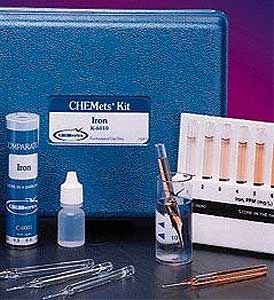
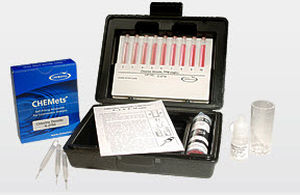
The DPD Method
References: USEPA Methods for Chemical Analysis of Water and Wastes, Method 330.5 (1983). APHA Standard Methods, 21st ed., Method 4500-Cl G (2005).
Potassium iodide is added to the sample before analysis. Ozone reacts with the iodide to liberate iodine. The iodine reacts with DPD (N, N-diethyl-p-phenylenediamine) to form a pink color. Results are expressed as ppm (mg/L) O3.
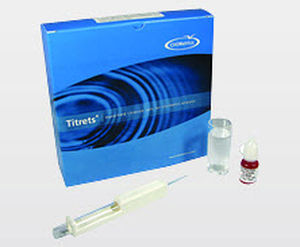
The Indigo Method
References: Bader H. and J. Hoigne, "Determination of Ozone in Water by the Indigo Method, " Water Research Vol. 15, pp. 449-456, 1981. APHA Standard Methods, 21st ed., Method 4500-03 B (2005).
With the indigo method, indigo trisulfonate reacts instantly and quantitatively with ozone, bleaching the blue color in direct proportion to the amount of ozone present. Malonic acid is included in the ampoule to prevent interference from up to 3 ppm chlorine. Results are expressed as ppm (mg/L) O3.
Related Searches
- Gas analyser
- Concentration analyser
- Liquids analyser
- Dust analyzer
- Portable analyser
- Water analyser
- Temperature analyser
- Trace analyser
- Carbon dioxide analyser
- PH meter
- Hydrogen analyser
- Portable pH meter
- Conductivity meter
- Photometer
- Chlorine analyser
- Process pH meter
- Nephelometer
- Ozone analyser
- Portable conductivity meter
- Portable photometer
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.