- Robotics - Automation - Industrial IT
- Industrial Software
- Monitoring software
- Delphin Technology AG
Management software Audit trailmonitoringmeasurementconfiguration
Add to favorites
Compare this product
Characteristics
- Function
- management, monitoring, measurement, configuration, server, acquisition, data archiving
- Applications
- alarm
Description
Validation of monitoring applications according to FDA 21 CFR Part 11 guidelines.
Data integrity through manipulation-proof measurement databases
The additional function Audit-Trail/FDA provides functions for the software ProfiSignal Basic or Klicks for the validation of monitoring applications according to the guidelines of FDA 21 CFR Part 11.
ProfiSignal projects are protected by the user management. Different authorisation levels can be assigned with a username and password entry. These range from simple observer to operator to administrator. Of course, individual operating functions can also be enabled or disabled for each user.
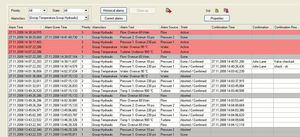
The audit trail logs user interventions depending on the user management, both in the case of configuration changes of the hardware as well as software changes and the actuation of operating elements on the PC. In case of project/software changes, the automatic versioning system is activated so that all documents are automatically available for validation.
Product highlights
User management with different authorisation levels
Integrated alarm management with rights-dependent provision of alarm lists and alarm acknowledgement
Redundant tamper-proof measurement data archiving on a measurement data server and directly in the acquisition hardware
Catalogs
Related Searches
- Automation software solution
- Management software solution
- Analysis software solution
- Process software solution
- Windows software solution
- Computer-aided design software
- Control software solution
- Online software
- Design software solution
- Monitoring software solution
- Measurement software
- Interface software
- Industrial software solution
- Quality software
- Visualization software solution
- Programming software
- Automated software
- Development software
- Network software solution
- Machine software
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.