When it comes to software, laboratories need a simple effective solution that is easy-to-use but provides the power to turn samples to knowledge. With the QSight™ Triple Quad LC/MS/MS Simplicity 3Q™ software, that's precisely what we aim to achieve. This modular flexible software architecture includes a collection of integrated modules that guide users through a workflow-based experience from method development through to results, flexible data viewing options for sample data processing to accredited reporting. Now, with Simplicity 3Q Version 3.0 software , this brilliant software has been built to meet the compliance guidelines of ISO 17025 laboratories.
Compliance
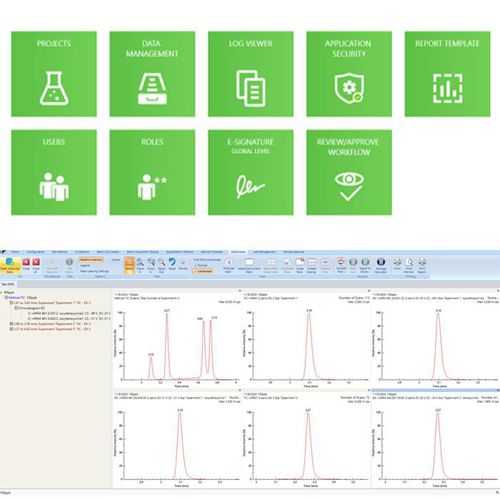
Simplicity 3Q streamlines regulated laboratory compliance activities, incorporating e-signature, user management, role management, project management, file versioning and approve/review features to support 21 CFR Part 11 and ISO 17025 regulations in one, easy-to-use platform. Additionally, managers can customize user access directly in the Simplicity 3Q environment, mitigating many unnecessary IT challenges.
Seamless Data Acquisition
Simplicity 3Q improves high throughput laboratory productivity by creating a streamlined workflow offering. Simplicities integration capabilities allow scientists to facilitate method development. Data can be reviewed by real time visualization allow timely intervention of experiments saving valuable samples and time to result.
Data Processing
Simplicity 3Q simple to use data processing module enables you to visualize all the key data parameters chromatogram integration, sample accuracy, and important calibration curves on a single screen.