- Machine-tools
- Machine Tool Accessory
- Production hopper
- Pharma Technology
Container hopper tabletfor production
Add to favorites
Compare this product
Characteristics
- Product applications
- for production, container, tablet
Description
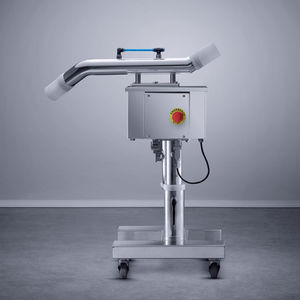
A quarantine buffering hopper can be installed between the tablet press and a large container on our iSeries machines to ensure quality of products.
Key benefits
• Continuous control of the product release in the container
• Reject of no conform batch portion
• Better product testing traceability
• Ideal for production in large size containers
• Central HMI with OPC UA capabilities
• Compact footprint
• CFR 21 Part 11 compliant and ATEX certified
A sampling gate delivers samples from the quarantine hopper to the tester. This sequence appears at least once in the buffer. The tester then analyses the quality of the products. If the product meets specifications, it is released into the product container via an automatic valve mechanism. If the product does not comply with the specs, the user receives an alarm signal and the small buffered quantity of products is rejected. An individual report, as well as a batch report, can be exported in Excel or PDF format and can then be analyzed and printed. The software follows CFR21 part 11 guidance. This solution is available in “DT” Dust Tight, “C” Containment ≤0EB4, and “HC” High Containment ≤0EB5 washable versions.
Catalogs
No catalogs are available for this product.
See all of Pharma Technology‘s catalogsRelated Searches
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.