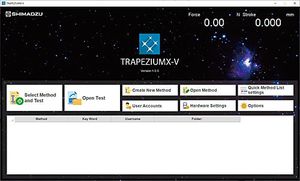
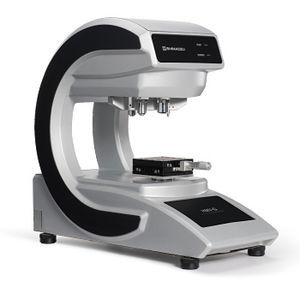
Integrated management software LabSolutions™ AGdatabasedata managementmanagement
Add to favorites
Compare this product
Characteristics
- Function
- management, analysis, data analysis, database, integrated management, data management
- Applications
- network, for pharmaceutical applications, medical
Description
Providing Confident, Secure Data Management on a Network System!
Supports Compliance with Regulations and Guidelines for Mechanical Characteristics Tests in the Pharmaceutical and Medical Equipment Industries
Secure, Confident Data Management
Mistakes are avoided thanks to database management
Robust security
Comfortable Operating Environment
Integrated management of a variety of analysis instruments
Quickly search through voluminous amounts of data
Heightened Management Productivity
Consolidated server-based management of users and other system information
Management of related information for each project
Total Support for Regulatory Compliance
Functionality supports CSV (IQ/OQ validation) activities
Supports document creation
The Autograph precision universal testing machines are now compatible with the latest in data integrity.
Connecting TRAPEZIUM X-V to the LabSolutions system, which provides ER/ES regulatory compliance, enables confident, reliable data management.
In addition to Autograph data, consolidated management is available for LC, GC, and UV data.
Data integrity refers to the assurance that all the data has been collected, and that it is free from defects or non-conformities. In other words, it is necessary to present not only the data itself but also the meta data (conditions settings, data analysis and other results of processes in which human hands have intervened) in a form that can be clearly seen, for verication together with the data. This is provided by Report Set.
Catalogs
No catalogs are available for this product.
See all of Shimadzu France‘s catalogsRelated Searches
- Automation software solution
- Management software solution
- Analysis software solution
- Windows software solution
- Computer-aided design software
- Control software solution
- Design software solution
- Monitoring software solution
- Interface software
- Measurement software
- Industrial software solution
- Quality software
- Development software
- Network software solution
- Test software
- EDM software
- Reporting software solution
- Optimization software solution
- Calculation software
- Data management software solution
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.