Management software UDIqualityreportingdatabase
Add to favorites
Compare this product
Characteristics
- Function
- management, quality, reporting, database, database, supply chain, labeling
- Applications
- process, medical
- Type
- centralized
Description
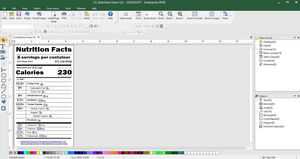
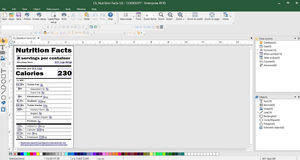
Easily create and print UDI-compliant labels
CODESOFT for UDI label creation
Health Industry Barcode (HIBC) and GS1 Databar barcode creation
Easy-to-use wizards
Create user rights and enable label security controls
TEKLYNX CENTRAL CFR for Label Management
Pull label elements from a centralized database for UDI-compliant labels
Automate labeling process with central management to ensure process enforcement and additional control
About UDI
Many organizations within the healthcare industry face challenges ensuring their barcode labeling systems are compliant with various regulations.
The Food and Drug Administration (FDA) requires that all medical devices distributed in the United States be labeled with a Unique Device Identifier (UDI), which is comprised of a unique numeric or alphanumeric code that is used to mark and identify medical devices within the supply chain.
As a result, all implantable, life-supporting/life-sustaining medical devices must bear a UDI label. Implementing UDI promises to reduce medical errors, simplify integration of data systems, and provide more rapid solutions to problems.
Full compliance with UDI includes three components:
Including the UDI in all applicable systems and procedures, including quality systems, medical device reporting, and corrections & removals
Accurate labeling of devices and packaging including all specified information
Loading the Device Identifier (DI), along with all associated information, into the Global Unique Device Identification Database (GUDID)
Catalogs
No catalogs are available for this product.
See all of TEKLYNX‘s catalogsRelated Searches
- Automation software solution
- Management software solution
- Analysis software solution
- Process software solution
- Windows software solution
- Control software solution
- Computer-aided design software
- Real-time software solution
- Online software
- Design software solution
- Interface software
- Quality software
- Automated software
- Development software
- Network software solution
- Test software
- Creation software
- Reporting software solution
- 2D software
- Safety software
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.