Control software FDA 21 CFR part 11managementcreationstorage


Add to favorites
Compare this product
Characteristics
- Function
- management, control, creation, storage, traceability, labeling
- Applications
- process, medical
- Type
- 2D
Description
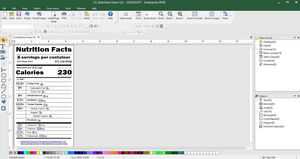
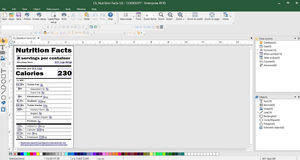
Regulatory compliance through label management
LABEL ARCHIVE for label Storage & security
Multi-stage approval of label designs by reviewers
Establish permissions to control access
Keep record of changes, comments, revisions, and print history of every label
TEKLYNX CENTRAL cfr for Label Management
Approve labels with electronic signatures in accordance with FDA 21 CFR Part 11
Custom reports show complete document history
Combines label creation, traceability, and print automation
FDA 21 CFR part 11 applies to the research, manufacturing, and distribution of medical products, and was established to protect the public health and ensure accuracy of electronic medical records. It also enables organizations to reduce costs by using electronic records in lieu of paper.
TEKLYNX CENTRAL CFR is TEKLYNX label management solution designed specifically to help organizations comply with FDA 21 CFR Part 11. It enables users to create complex barcodes such as the 2D DataMatrix barcode, Health Industry Bar Code (HIBC), GS1 Databar, and more. Ensure a secure and compliant process by establishing user permissions and applying electronic signatures to your labels throughout their entire lifecycle.
VIDEO
Catalogs
No catalogs are available for this product.
See all of TEKLYNX‘s catalogsRelated Searches
- Automation software solution
- Management software solution
- Analysis software solution
- Process software solution
- Computer-aided design software
- Windows software solution
- Control software solution
- Online software
- Real-time software solution
- Design software solution
- Interface software
- Quality software
- Automated software
- Development software
- Network software solution
- Test software
- 2D software
- Creation software
- Reporting software solution
- Safety software
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.