Supply chain software DSCSAdesigndata exchangetracking

Add to favorites
Compare this product
Characteristics
- Function
- design, tracking, traceability, data exchange, supply chain
- Applications
- label printing
- Type
- 2D
Description
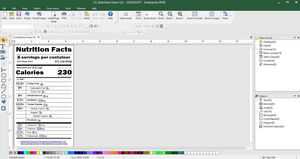
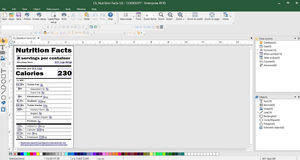
Easily identify and trace prescription drugs
CODESOFT for label design & serialization
Add product identifiers and 2D barcodes to your labels with intuitive GS1 barcode wizards
Create custom forms for label printing to protect designs and standardize data entry
Create user rights and enable label security controls
The Drug Supply Chain Security Act (DSCSA) aims to build an electronic, interoperable system to accurately trace prescription drugs throughout the supply chain as they are distributed in the United States. Its goal is to verify legitimate products, detect suspect and illegitimate products, and better facilitate drug product recalls protecting consumers from counterfeit, contaminated, or otherwise harmful products.
The FDA has outlined tiered compliance deadlines for DSCSA implementation, beginning with prescription drug manufacturers by November 27, 2017. Repackagers were required to reach compliance by November 27, 2018, followed by wholesale distributors by November 27, 2019, and dispensers by November 27, 2020. The final deadline for serialization and traceability for DSCSA was November 27, 2023. The FDA announced a one-year delay in enforcing the electronic tracking requirement under the DSCSA. The enforcement of an interoperable package-level electronic data exchange has now shifted to November 27, 2024.
DSCSA requires a unique product identifier at the prescription drug package level that consists of:
National Drug Code (NDC)
Serial number
Lot number
Expiration date
Product identifier in 2D barcode format
VIDEO
Catalogs
No catalogs are available for this product.
See all of TEKLYNX‘s catalogsRelated Searches
- Automation software solution
- Management software solution
- Analysis software solution
- Process software solution
- Windows software solution
- Computer-aided design software
- Control software solution
- Real-time software solution
- Online software
- Design software solution
- Interface software
- Quality software
- Automated software
- Development software
- Network software solution
- Test software
- 2D software
- Creation software
- Reporting software solution
- Safety software
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.